
The world is filled with matters. Just one quick look around us is enough to see how natural things such as water, air, and rocks, as well as artificial things such as plastic and vinyl are all made of matters. What will happen if we keep breaking matters into smaller pieces? If you divide them to the point that they cannot be divided anymore, it is called atom—the basic unit of matter.
A pure substance of one kind of atom is called an element. About 110 elements have been discovered, and about 90 of them are found in nature. On top of that, those that can be commonly found are only about 40.
The mystery of chemical bonding
In one theme park, there is a village that was made with small colorful blocks. It is amazing that large buildings that are considered landmarks, and cars, and even swings in the playground can be made by stacking blocks. Likewise, most of substances around us are compounds from the combination of two or more elements. Just as several types of blocks are stacked to form various shapes, atoms combine to form various compounds.
Interestingly, the properties of the compounds differ significantly from those of the constituent elements. Water molecule (H₂O) is a compound in which two hydrogen atoms and one oxygen atom meet and bond. Hydrogen (H₂), which is mainly in the form of a molecule by bonding two atoms, is a gas that is easy to burn, and is easily ignited when in contact with the air. Oxygen (O₂), which is a diatomic molecule like hydrogen, is highly reactive and so it combines directly with almost all elements. Water which is formed by bonding these two substances is normally in the state of liquid and is very stable. It is used to extinguish fire rather than burning other materials like oxygen or burning on its own like hydrogen.
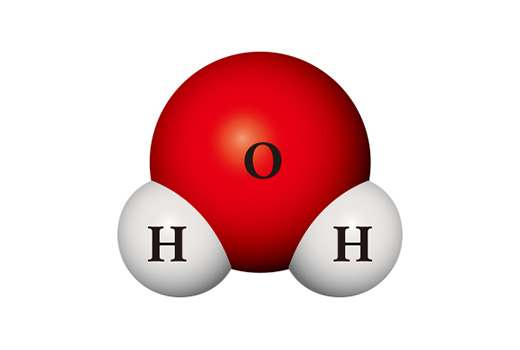
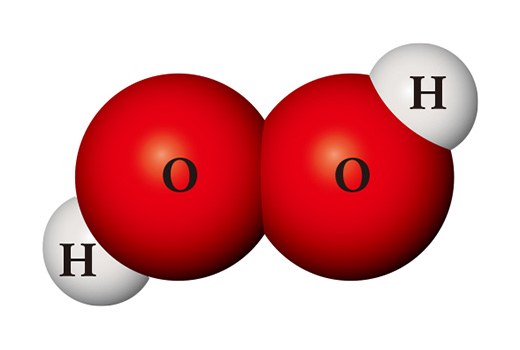
A completely different substance can be made from the same kinds of atoms. When two hydrogen atoms and two oxygen atoms meet, they become hydrogen peroxide (H₂O₂) which is used as a disinfectant. Hydrogen peroxide is so unstable unlike water that it is easily decomposed into water and oxygen at room temperature.
Just as you can make various things by using the same shape and size blocks, various substances can be formed even by only one type of element. Among them, carbon is unique. Both diamond and graphite are made of only carbon, but they have completely different properties due to different bonding arrangements.
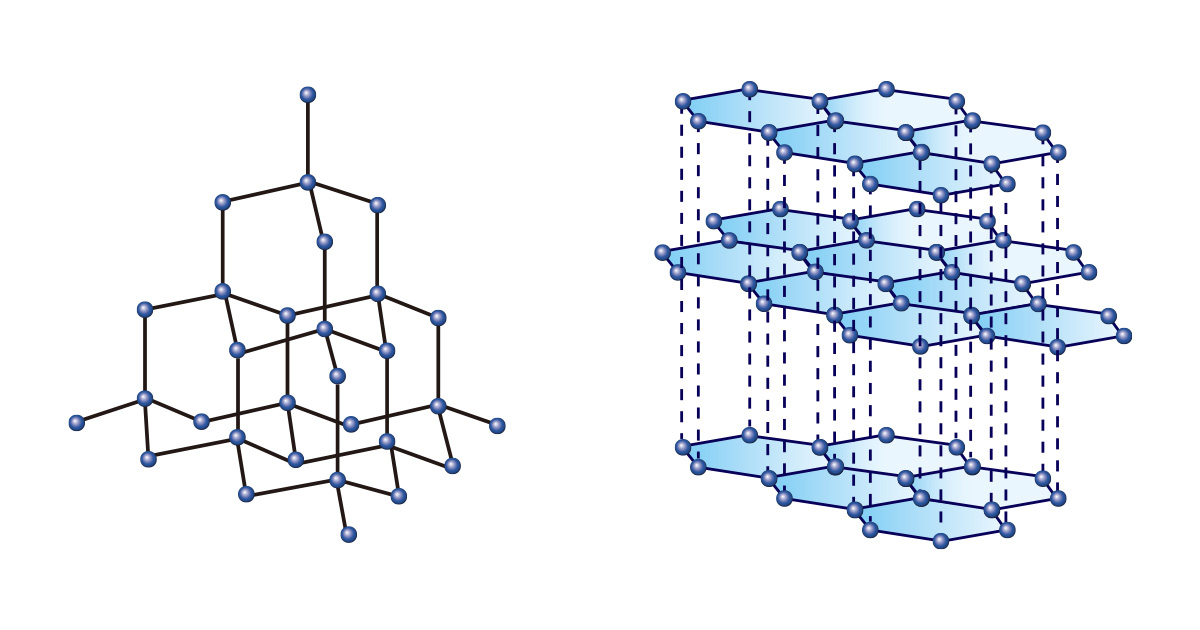
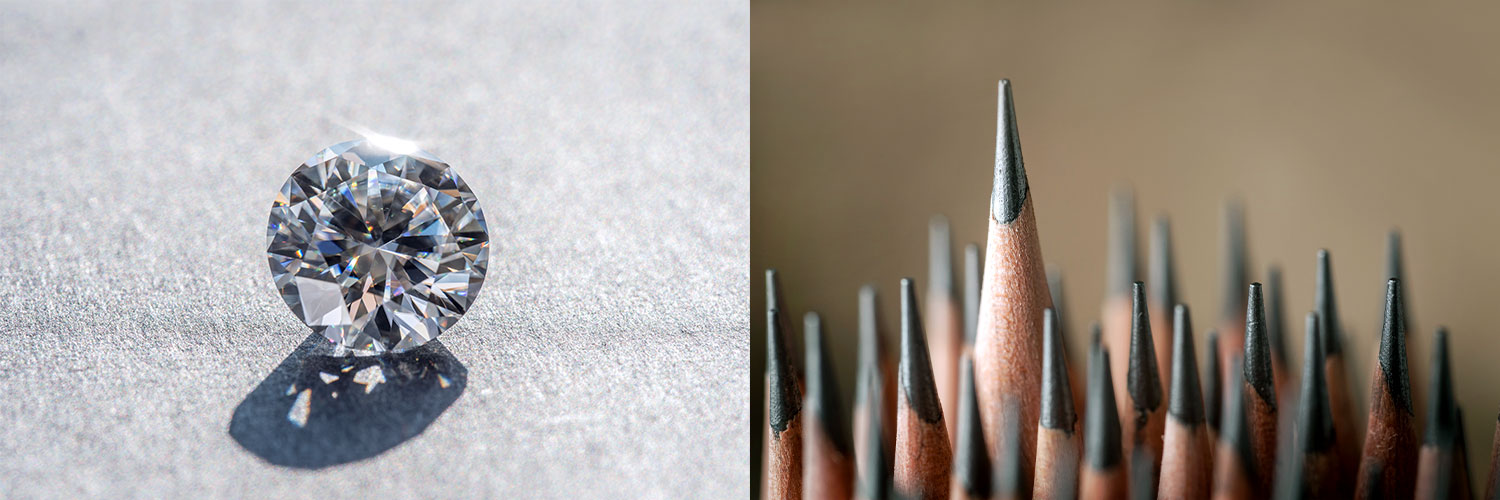
Diamonds, in which carbon atoms are continuously bonded by forming a tetrahedral structure, are so hard that they are not only regarded as gems representing eternity, but also used for cutting or polishing materials in the industrial field. On the other hand, graphite which is in the form of stacked layers of hexagonal two-dimensional structures is used as a pencil lead because it is soft and easy to draw with.
Rules for making compounds
There are certain rules in which atoms form compounds. An atom consists of a positively charged nucleus surrounded by one or more negatively charged particles called electrons. The nucleus of an atom contains protons and neutrons. Since the number of protons and electrons contained in an atom is the same, the atom itself is electrically neutral.
Scientists assume that electrons are layered around atomic nucleus according to the energy level in order to simply distinguish the energy state that the electrons take. The layer formed by electrons is called an electron shell, and there is a designated number of electrons for each shell: 2 electrons in the first layer, 8 electrons in the second layer, and 18 electrons in the third layer.
Among them, electrons in the outermost shell participate in chemical bonding. Atoms have a tendency to have eight electrons in the last shell even if they have to lose or gain electrons to do so, and this is called the octet rule. For example, oxygen with 8 electrons contains 2 electrons in the first shell and 6 electrons in the second shell. Therefore, in order for the last shell to have 8 electrons, chemical bonding is performed to obtain 2 more electrons.
Various chemical bonds
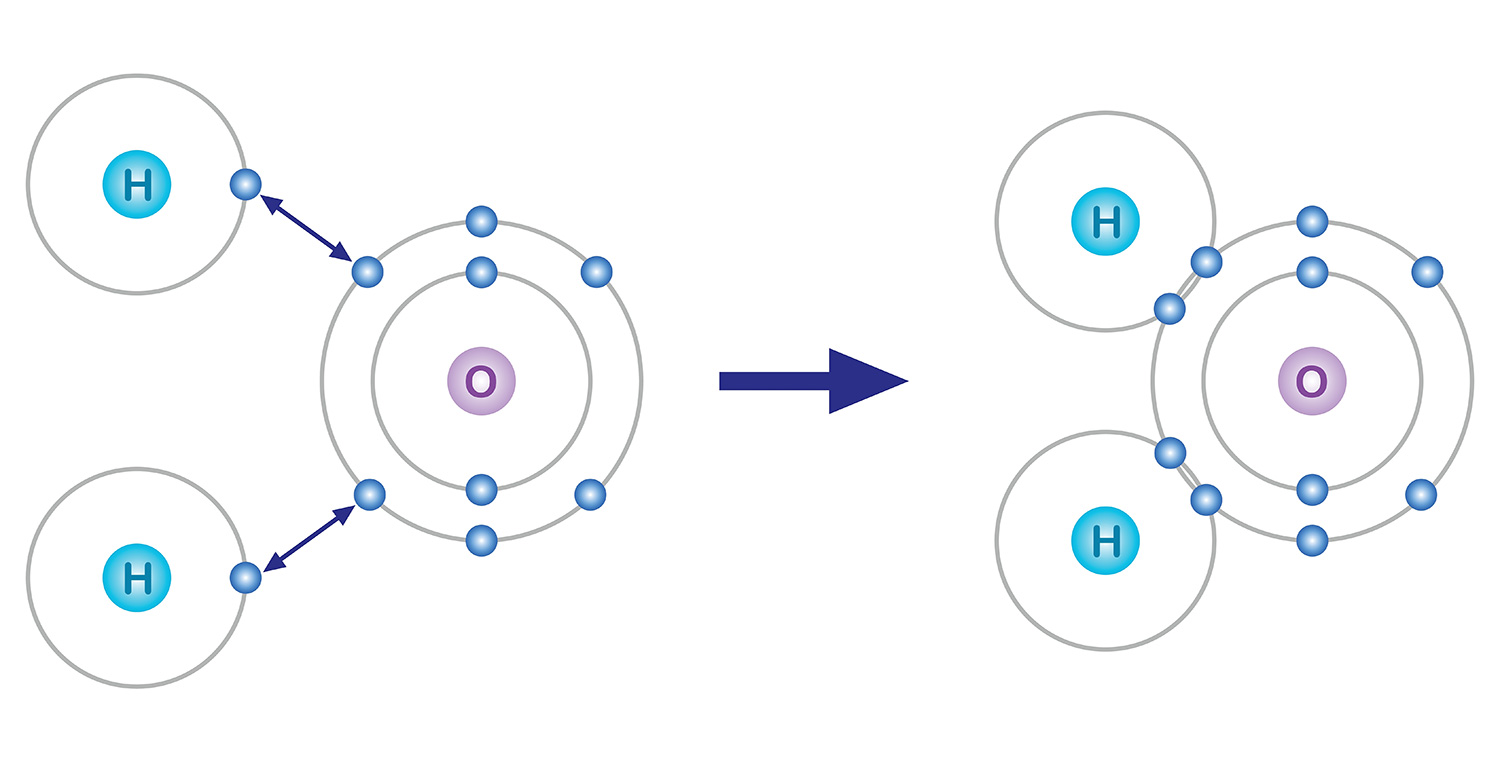
There are various ways in which an atom combines with other atoms. One of them is the covalent bond, a win-win strategy where atoms satisfy the octet rule by sharing each other’s electrons as if they are holding each other’s hands; the particles created by the combination of atoms are called molecules.
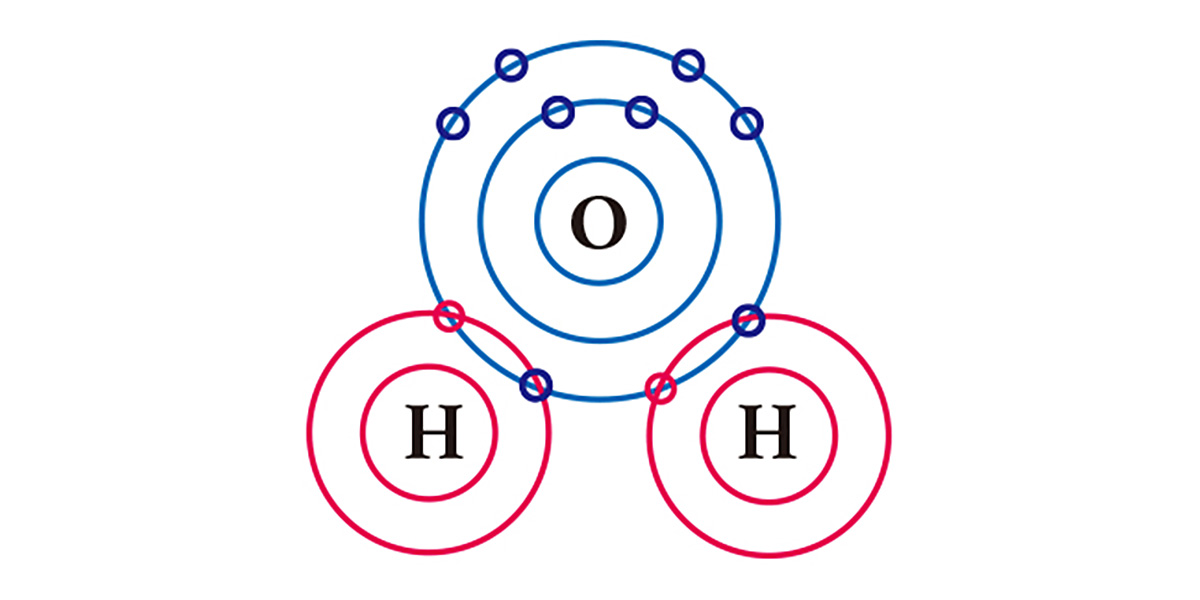
Take water for example. An oxygen atom, which has 6 electrons in its outermost electron shell, lacks 2 electrons and an hydrogen atom, which has one electron, lacks one. So in order to be stabilized, an oxygen atom shares the two electrons of the two hydrogen atoms in a covalent bond, and they form one molecule. Both the oxygen atom and the hydrogen atoms basically got two electrons each by sharing one electron each.
As for carbon dioxide (CO₂), the carbon atom, which requires four more electrons to be stabilized, combines with two oxygen atoms to get four electrons (two from each). The bond between carbon and oxygen is much stronger than the bond between oxygen and hydrogen in the water molecule. Just as two hands holding each other are stronger than one hand, since the number of electrons that they share are higher, its bond is stronger.
Among the elements, there are ones that like to give generously. Metals such as sodium, aluminum, and iron fulfill the octet rule and easily release their remaining electrons. Conversely, nonmetals such as oxygen, nitrogen, and chlorine prefer to receive electrons rather than to give them. The bond between them is called ionic bonding, which is an electrostatic bond in which the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
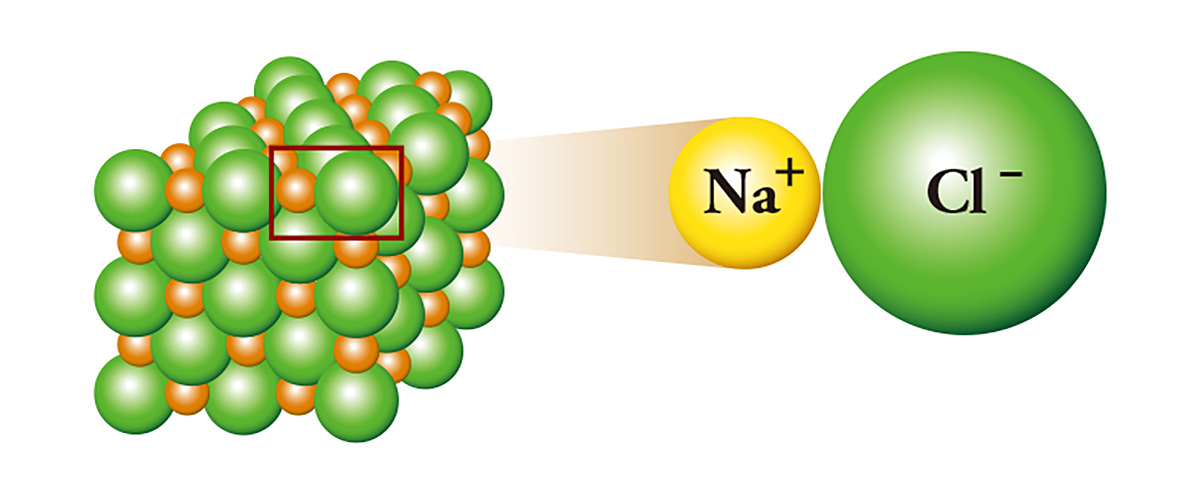
Sodium chloride (NaCl), commonly referred to as salt, is a representative of ion-binding; sodium, which has one extra electron, binds with chlorine by giving the electron to the chlorine which lacks one electron. Sodium that gives the electron becomes a cation, and chlorine that receives the electron becomes an anion. As a result, an electrical attraction occurs between the two ions and they stick to each other like the north pole and the south pole of a magnet, and form crystals. Sodium, a metal that is highly reactive enough to be stored in mineral oil or kerosene, and chlorine, a toxic substance that is used as a poison gas, meet and become salt.
Metallic bonds can be seen in solid metals composed of one type of atom, such as gold or aluminum. It is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations; the delocalized electrons move freely between the cations and maintain the bond.
Atoms bond by sharing or exchanging electrons. Sometimes dangerous substances come together to form a compound more stable than any other compounds, and depending on how they are combined, they become weaker or stronger. In the world of particles, atoms are compounded with one another and form completely different matters.
Most matters consist of only about 20 elements. However, when these few elements are combined together, many different compounds can be made. According to Chemical Abstract Service [CAS], there are more than 140 million chemicals on Earth, and more than 10,000 new compounds are being registered every day (as of April 2018). Given that there are still many that have not been discovered, the number of chemicals will be even more. The mystery of the micro world, which is creating an innumerable variety of compounds, is truly mysterious.